What is a chelate
The word ‘chelate’ is derived from the Greek word meaning ‘claw’. Many of the micro elements such as Fe, Mn, Cu and Zn are difficult for the plant to absorb and in some cases, deficiency symptoms may occur if these micro-elements are not made available to the plant in chelated form. A chelate is a relatively large soluble organic molecule which increases the solubility of the metal ions. Chelates have been extensively used in soils in order to overcome deficiency symptoms of various metals such as iron and manganese. The chelating agent is the large organic molecule and protects the micro element (metal ions) from oxidation and precipitation into unavailable form. The chelating agent binds the micro elements through ligand bonds. It is important to note that B and Mo cannot be bound into chelates and are absorbed in other forms.
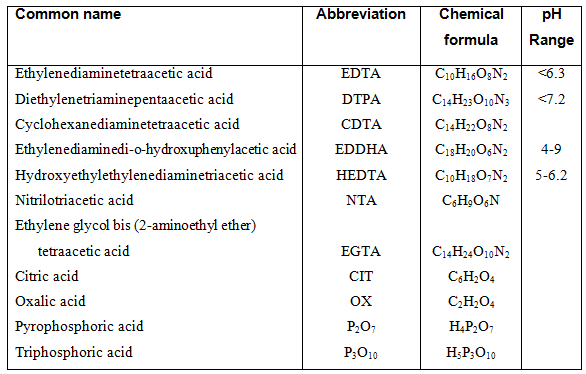
The details through which chelates bind ions are complex and will not be discussed, but what is important is to know which type of chelated to use. Chelates differ in their stability at various pH ranges. The stability of chelates are (from highest to lowest): EDDH>DTPA>EDTA>HEDTA>citrate. EDDHA is the most stable chelate but is also the most expensive. In hydroponic systems the most common chelates are EDTA, DTPA and HEDTA. These chelates are effective only if the pH of the nutrient solution is below 7.2. If the pH rises above 7.2 the stability decreases up to 90 %. Fe-EDTA is stable below a pH value of 6.3 after which the solubility of Fe decreases 1,000 times. Above pH of 6.5, Fe-DTPA competes with Ca2+, after which Ca2+ is bound to the chelate and Fe becomes unavailable to the plant. There are many such interactions between the various chelates and micro elements, however, the important aspect is to use the correct chelate within a pH range.